CRISPR screening reveals H2AZ1 as a key driver of mesenchymal stem cell aging
GA, UNITED STATES, June 17, 2025 /EINPresswire.com/ -- In a new discovery that may reshape anti-aging strategies, researchers have identified the histone variant H2A.Z variant histone 1 (H2AZ1) as a pivotal driver of cellular aging in human mesenchymal stem cells (hMSCs). By leveraging CRISPR-based genomic screening, the research team found that depletion of H2AZ1 can reverse cellular aging hallmarks and rejuvenate stem cell function. Conversely, its overexpression accelerates the aging process. The study unveils a novel epigenetic mechanism: H2AZ1 silences the secreted growth factor pleiotrophin (PTN), which is critical for tissue repair and hippocampal neurogenesis, by binding to its five enhancer regions (E1–E5), offering a new therapeutic target to delay aging and enhance regenerative medicine.
Cellular senescence—a state of irreversible growth arrest accompanied by chronic inflammation—is a major contributor to aging and tissue degeneration. Stem cells, which are essential for tissue maintenance and repair, progressively lose their regenerative capacity with age. One underexplored aspect of this decline is the role of chromatin remodeling, especially histone variants like H2A.Z variant histone 1 (H2AZ1), which replace conventional histones to regulate gene expression. While changes in chromatin have long been linked to aging, the specific impact of individual histone variants has remained unclear. Premature aging diseases such as Werner syndrome and Hutchinson–Gilford progeria highlight the urgent need to uncover epigenetic regulators driving stem cell senescence.
Published on June 19, 2024, in Protein & Cell, the letter-style study led by researchers from the Chinese Academy of Sciences, etc. spotlights H2AZ1 as a key pro-senescence factor in hMSCs. Through an expansive CRISPR screen, the team revealed that silencing H2AZ1 boosts PTN expression and reverses aging phenotypes, whereas H2AZ1 overexpression intensifies senescence. Using chromatin mapping and functional assays, they confirmed that H2AZ1 suppresses PTN by directly binding to its enhancers. This study provides a molecular blueprint to understand—and potentially reverse—stem cell aging.
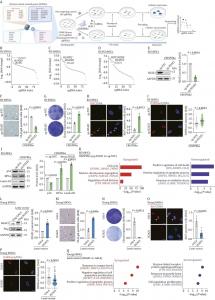
The researchers screened 32 histone variants across three stem cell aging models: natural replicative senescence, Werner syndrome, and Hutchinson–Gilford progeria. H2AZ1 consistently emerged as the top candidate, with its knockout reducing senescence biomarkers such as SA-β-gal and p16, and restoring cell proliferation. Transcriptome analysis showed that H2AZ1 deletion upregulates cell-cycle genes (e.g., FOXM1, LMNB1) and downregulates oxidative stress pathways, restoring stem cell vitality. Chromatin immunoprecipitation (ChIP)-seq confirmed H2AZ1’s direct binding to PTN enhancers—regions that regulate the expression of this neurogenic and reparative growth factor, and luciferase reporter assays further demonstrated that H2AZ1 suppresses enhancer activity of PTN. PTN knockout mimicked H2AZ1-driven senescence, while PTN overexpression rescued aging effects. Critically, H2AZ1’s role persisted under ultraviolet (UV) radiation, oxidative stress (H₂O₂), and oncogene (H-RasV12) transduction, underscoring its broad relevance.
Dr. Guang-Hui Liu, one of the study’s corresponding authors, said: Our study bridges a critical knowledge gap in how histone variants regulate stem cell aging. Dr. Jing Qu, another co-corresponding author added: By identifying H2AZ1 as a suppressor of PTN, we’ve uncovered a druggable axis that could transform therapies for age-related diseases. This CRISPR screening platform offers a powerful tool to discover new geroprotective factors, guiding future anti-aging interventions. Their findings point to H2AZ1 as a master regulator of senescence with therapeutic potential.
Looking ahead, the H2AZ1-PTN axis offers a twofold promise: a biomarker of biological aging and a target for regenerative treatments. Strategies to inhibit H2AZ1 or elevate PTN could delay stem cell exhaustion in conditions such as osteoarthritis or premature aging syndromes. Notably, PTN levels decline in aged cells and tissues, aligning with its role as a rejuvenation factor. The study’s CRISPR screening framework paves the way for systematic exploration of other epigenetic regulators. Given PTN’s known roles in neural and muscular repair, this mechanism may also extend beyond mesenchymal stem cells to other tissues. Clinically, small molecules modulating H2AZ1 activity might synergize with senolytic therapies. While further in vivo validation is essential, this research marks a critical step toward epigenetic-based solutions for healthier aging.
References
DOI
10.1093/procel/pwae035
Original Source URL
https://doi.org/10.1093/procel/pwae035
Funding information
This work was supported by the National Natural Science Foundation of China (82125011, 81921006, 92149301, 82322025, 82122024), the National Key Research and Development Program of China (2020YFA0804000, 2022YFA1103700, 2020YFA0112200, 2021YFF1201000, the STI2030-Major Projects-2021ZD0202400), the National Natural Science Foundation of China (92168201, 82330044, 32341001, 92049304, 92049116, 32121001, 82192863, 82071588, 82361148130, 82361148131, 82271600), CAS Project for Young Scientists in Basic Research (YSBR-076, YSBR-012), the Program of the Beijing Natural Science Foundation (Z230011), the Informatization Plan of Chinese Academy of Sciences (CAS-WX2022SDC-XK14), New Cornerstone Science Foundation through the XPLORER PRIZE (2021-1045), Youth Innovation Promotion Association of CAS (E1CAZW0401, 2022083), Excellent Young Talents Program of Capital Medical University (12300927), The Project for Technology Development of Beijing-affiliated Medical Research Institutes (11000023T000002036310), Excellent Young Talents Training Program for the Construction of Beijing Municipal University Teacher Team (BPHR202203105), and Young Elite Scientists Sponsorship Program by CAST (2021QNRC001).
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.